Road Salt Usage Quiz

Test your knowledge with the questions below and find out if you could be using less salt and helping to protect the watershed without compromising the safety of you and your family!
Although Spring has officially sprung, Winter isn’t one to go away without a fight (we’ve all seen it snow in April or even May), so the days of icy pavement are not yet behind us, and that means road salt is going to continue to be an important factor in public safety for the time being.
But with warmer weather on the way, the melting snow and ice will be flowing into our beautiful river and streams, carrying with it everything that’s been trapped within through the winter, so it’s not too late to be thinking about how much salt you’re using to de-ice your driveways and sidewalks. It’s a great time to ask yourself: how much salt is actually needed to clear the ice?
How many tablespoons of salt per meter squared?
How many tablespoons of salt do you think you would need to de-ice 1 meter squared (approximately one section of a sidewalk)?

8 tablespoons
Try again!
Way too much salt!
7 tablespoons
Try again!
6 tablespoons
Try again!
5 tablespoons
Try again!
4 tablespoons
Try again!
3 tablespoons
Try again!
2 tablespoons
Correct!
According to Conservation Ontario, it takes up to 200 kg of dry salt to de-ice 1 km of a 2-lane road, which works out to just 30 g (2 tablespoons) per square meter! To put it another way, that’s a ball of salt just a little bigger than a golf ball.
1 tablespoon
Try again!
That's a bit too little.
Let’s try a more real-world, relatable example.
How long will a 20-kg bag of salt last?
Let’s say you just bought a standard, 20-kg bag of road salt from the local hardware store. How many times do you think you can completely de-ice a 1-car driveway before that bag runs out?
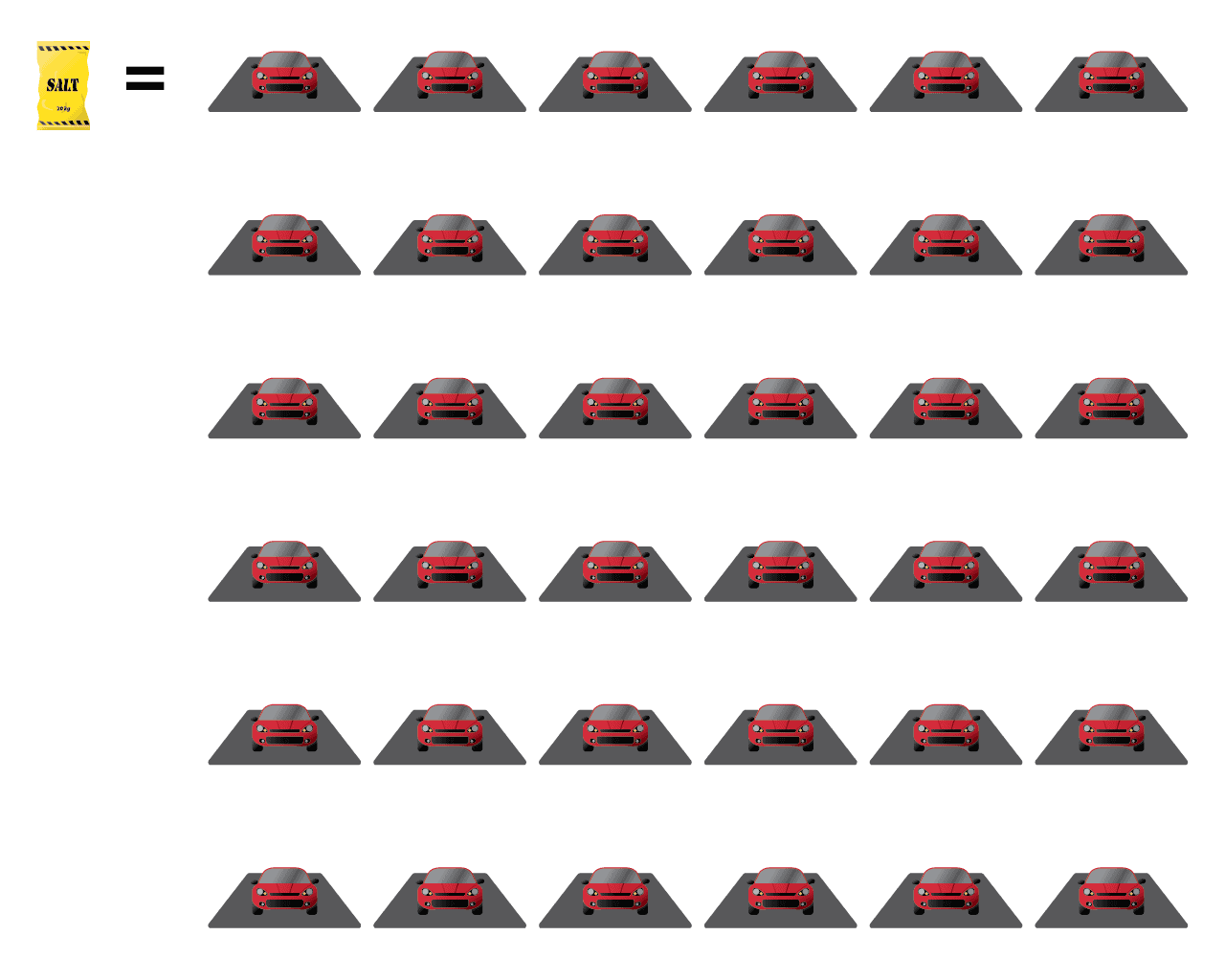
36 times
Try again!
Not quite that many times.
30 times
Correct!
One 20-kg bag of road salt is enough to de-ice a 1-car driveway 30 times! That means if you had freezing rain every single day, one single bag of salt would still last you an entire month!
24 times
Try again!
18 times
Try again!
12 times
Try again!
6 times
Try again!
You can spread it out much more than this!
So what does that look like to scale, as a single area?
What area will a 20-kg bag of salt cover?
Here is an NBA basketball court with that same 20-kg bag of salt in the corner for scale. Click the area of the basketball court that you think could be de-iced by that one bag.
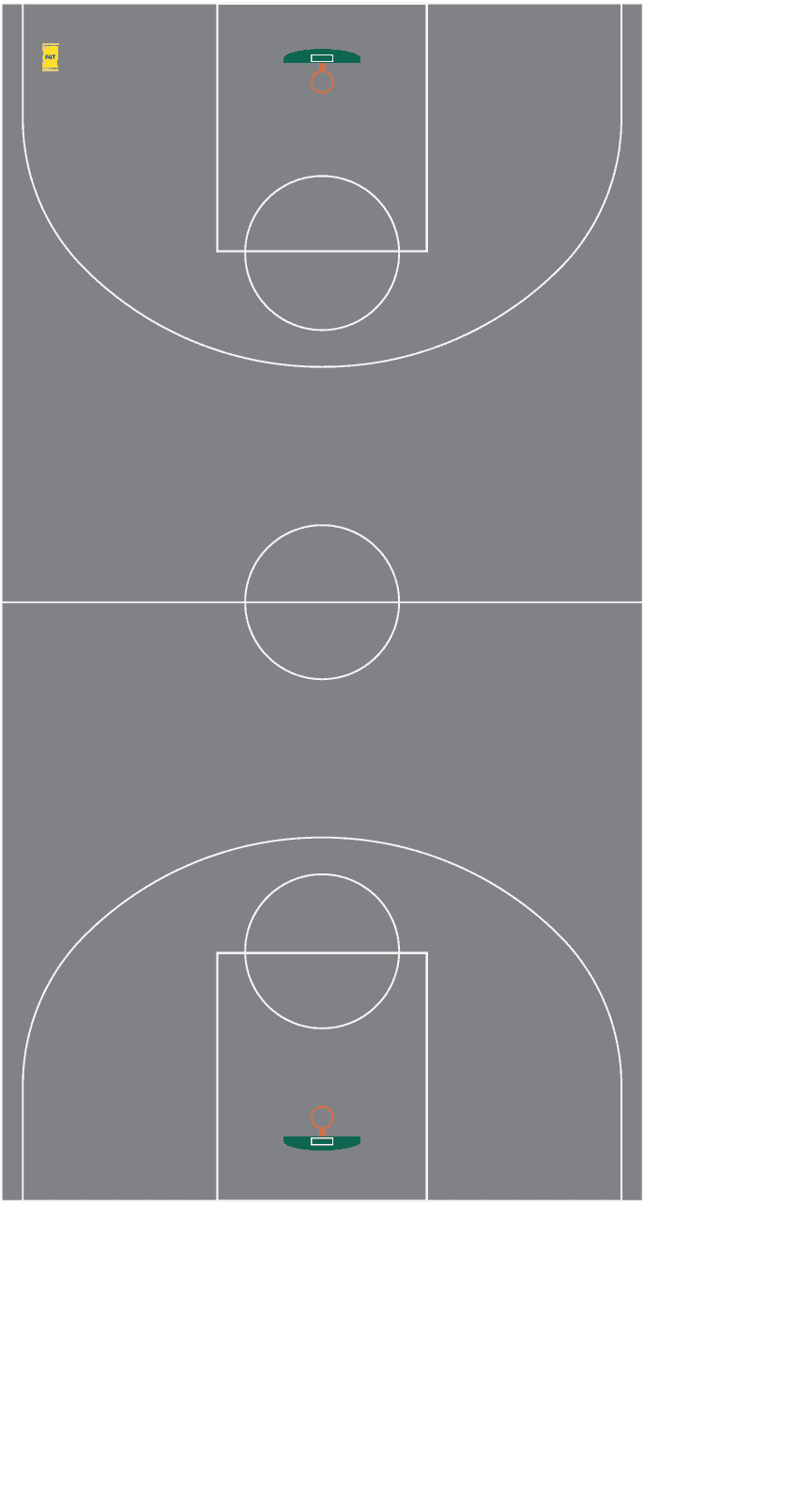
More than a whole basketball court
Correct!
One 20-kg bag of road salt is enough to de-ice almost 670 square meters! That’s over 1.3x the size of a basketball court!
The whole basketball court
Try again!
Think outside the box on this one!
Half of the basketball court
Try again!
You can cover even more than this!
Area inside the 3-point line
Try again!
Area inside the freethrow line
Try again!
Area of the center circle
Try again!
You can cover much more than that!
Now that you know how much salt should be used for de-icing pavement, think about how much salt you see on the sidewalk, on roads, or in parking lots. Is the right amount of salt being used? In most cases, probably not, and we believe it’s having negative impacts on our watershed.
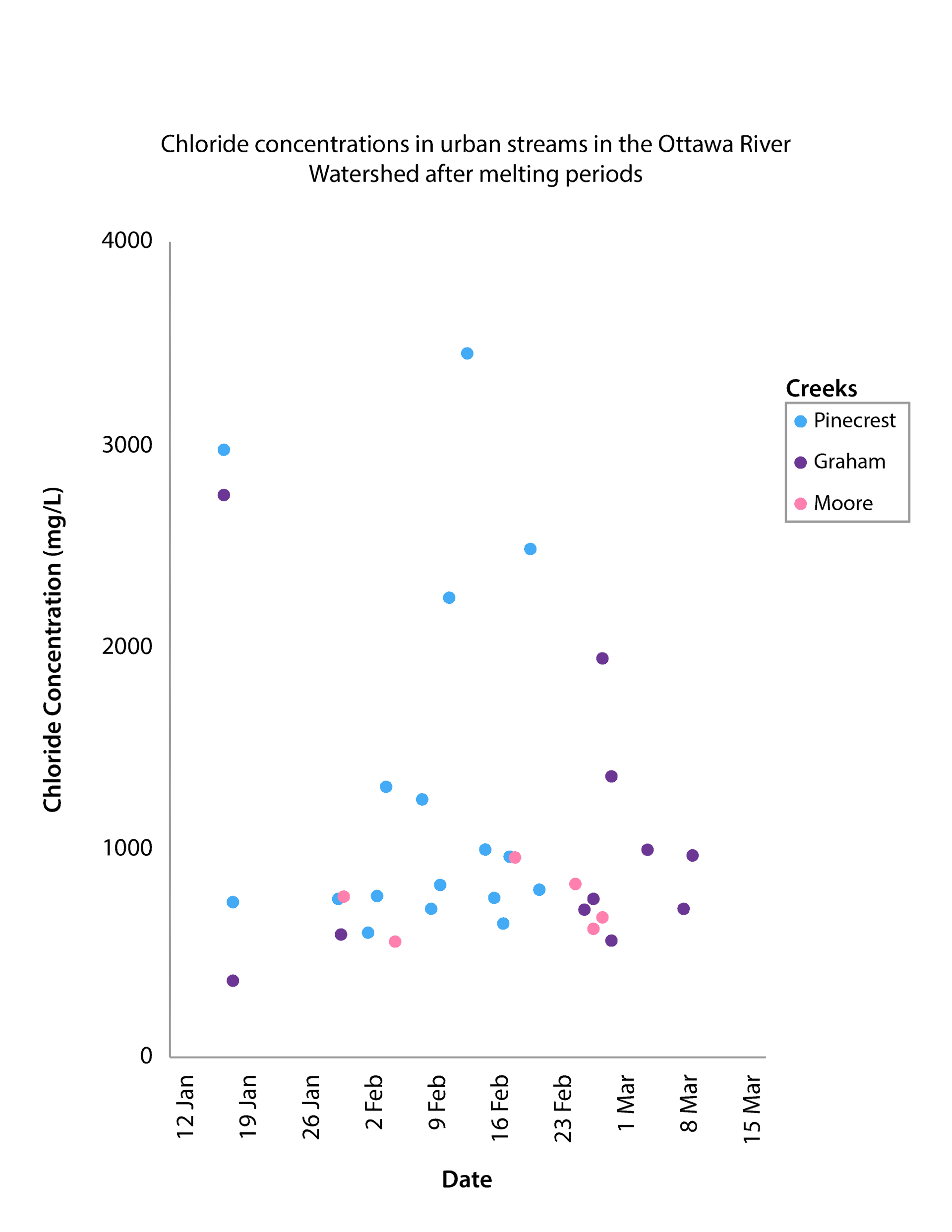
In February this year, Ottawa Riverkeeper started a pilot study to monitor the effects of road salt use on our river. Some of our amazing volunteer citizen scientists have been collecting water samples in a few select urban streams so that we can measure the concentration of chloride in the water, and the results have been shocking!
To give you an idea of where to start, seawater has a chloride concentration of around 19000 mg/L. Brackish water in tidal estuaries may have chloride levels between 500 and 5,000 mg/L.
What did Ottawa Riverkeeper’s salt monitoring results look like?
Click the areas of the graph area below to guess the average, maximum, and minimum concentrations in the 3 streams we’ve sampled so far.
0 to 300 mg/L
Try again!
300 to 700 mg/L
Correct!
You got the minimum value. The lowest concentration of chloride in an urban stream measured by Ottawa Riverkeeper so far this year was 375 mg/L in Graham Creek on January 17th.
700 to 1000 mg/L
Try again!
1000 to 1300 mg/L
Correct!
The average concentration of chloride in urban streams as measured so far this year by Ottawa Riverkeeper is 1145 mg/L
1300 to 1700 mg/L
Try again!
1700 to 2000 mg/L
Try again!
2000 to 2300 mg/L
Try again!
2300 to 2700 mg/L
Try again!
2700 to 3000 mg/L
Try again!
3000 to 3300 mg/L
Try again!
3300 to 3700 mg/L
Correct!
The highest concentration of chloride in an urban stream measured by Ottawa Riverkeeper so far this year was 3454 mg/L in Pinecrest Creek on February 12th.
3700 to 4000 mg/L
Try again!
Great work! But there’s still something missing. All that data is great, but it doesn’t tell us much until we can put it into context. Based on our data (plotted in the graph below), what do you think the acceptable concentration of chloride is in freshwater streams like the 3 we sampled from?
At what concentrations does chloride become toxic to freshwater organisms?
Click the concentrations at which you think chloride becomes toxic to freshwater organisms in the short and long term (acute and chronic).
120 mg/L
Correct!
The chronic toxicity threshold for chloride in freshwater is 120 mg/L. That means that chloride can be toxic to freshwater organisms if its concentration stays above 120 mg/L for extended periods.
As you can see, most of our samples exceeded this threshold.
640 mg/L
Correct!
The acute toxicity threshold for chloride in freshwater is 640 mg/L. That means any concentration above 640 mg/L can be toxic to freshwater organisms, even if it only lasts for a short period of time.
As you can see, many of our samples exceeded this threshold.
1000 mg/L
Try again!
1370 mg/L
Try again!
2160 mg/L
Try again!
2980 mg/L
Try again!
3500 mg/L
Try again!
Shocking, right? Since the Ottawa River and its tributaries are naturally freshwater ecosystems, our data suggests that many native aquatic organisms in these 3 streams have been negatively impacted, at least during the time of year when road salt use is at its peak.
Help us protect our beautiful river and watershed by challenging yourself and others to be #LessSalty!
Bonus Question!
30 g per 1 meter squared is the right amount of salt for de-icing pavement that is between 0 and -7°C. Above 0°C, ice melts itself, so no need for salt, but what if it gets colder? How much more salt do you think you should add as pavement cools below -7°C?
0 g/m squared (same amount)
Try again!
1 g/m squared
Try again!
3 g/m squared
Try again!
5 g/m sqaured
Try again!
10 g/m squared
Try again!
None of the above
Correct!
Salt works as a de-icer by lowering the melting temperature of ice from 0°C to -7°C, so if the ice is -8°C or below, adding salt won’t make it melt. What’s worse is if the temperature drops below -7°C after you’ve used salt to melt some snow or ice, it can refreeze and make things even slipperier! So check the forecast, and if it’s going to be too cold for salt, try an anti-slip alternative like sand or grit, at least until things warm up a little.
